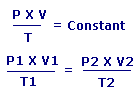The Combined Gas Law is a gas law which combines Charles's law, Boyle's law, and
Gay-Lussac's law.
Charles's law states that volume and
temperature are directly proportional to each other while pressure is held constant. Boyle's
law asserts that pressure and volume are inversely proportional to each other at fixed
temperature. Finally Gay-Lussac's law introduces a direct proportionality between
temperature and pressure at constant volume. The inter-dependence of these variables is
shown in the combined gas law.
Combined Gas Law states that:
The ratio between the Pressure-volume constant and Temperature of a system remains constant.
|